Which of the Following Elements Has the Smallest Atomic Radius
Which of the following elements has the smallest atomic radius bromine selenium chlorine sulfur. The smaller of four is the smallest and the smaller the ball so its a positive number with the smallest.
Solved A In The Following Set Which Atom Has The Smallest Chegg Com
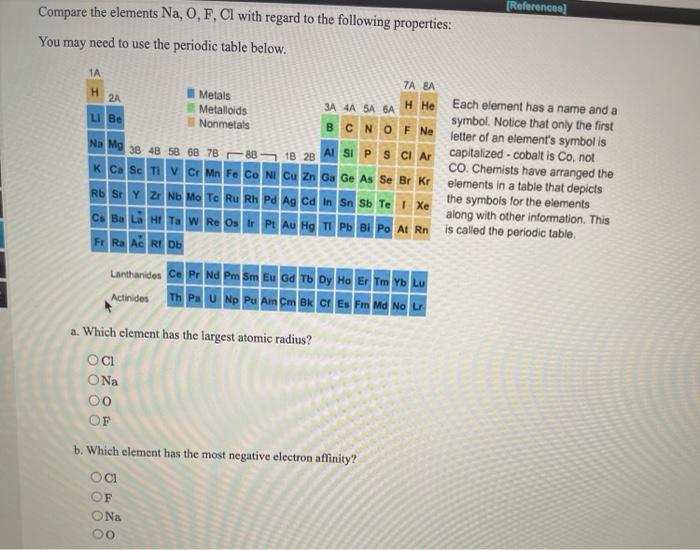
As you know atomic size increases as move move down a group of the periodic table.
. Nitrogen atomic radius. Chlorine has the smallest atomic radius and the highest ionization energy. Calcium potassium scandium titanium Which of the transition metals has the smallest atomic radius The atomic mass of titanium is 4788 atomic mass units.
Which of the elements listed below has the greatest atomic radius. Which element has the smallest atomic radius. Since The element which will in the right most of the Theriodic table will be having smallest alomic radius.
An atom of which of the following elements has the smallest atomic radius. Cl Br F O Al C Li Cs and Xe The element which has the smallest radius. Since the elements listed are all in the same group iodine would have actually the best atomic radius due to the fact that it farther down the period compared come the others.
That atomic size should decrease across a Period from left to right as we face the Table can be attributed to the increase of nuclear charge ie. Which of the following has the smallest atomic radius so that the elements have a lie and 10 in same period have been read every ko down the group Justdial of chlorine we have chlorine block to halogen family and similarly we not is we have iron sorry we have potassium potassium somewhere around latest. Asked Sep 3 2019 in Chemistry by Emagee.
119 rows Atomic number Elements Atomic Radius of Elements pm 1. Thus the order of size is NaMgBN. This atomic mass represents the.
From among the elements choose the following. Which of the following statements is true about. The right most comer of perioder and it belongs to Group 17 and we know that across the period aromir rodres decreases hence correct option is i.
Atomic radius of Hydrogen H. In the case of halogens which are group 17 elements fluorine F will have the smallest atomic radius of the group. Atomic radius rises down each group of the regular table and toward the left of every period.
The atomic size decreases towards the right in a period as effective nuclear charge increases. Which of these elements is the smallest atom and which has the highest ionization energy. Which of the following elements has the smallest atomic radius.
B Al S P Si b. Hence the smallest is NNitrogen. Which of the following elements has the smallest atomic radius.
Which of the following elements has the smallest ionization energy. The atomic radius decreases as we move from left to right along the period. If you exclude astatine At which is radioactive iodine I will have the largest atomic radius of the group.
An atom of which of the following elements has the smallest atomic radius. Sulfur Chlorine Selenium Bromine See periodic table. Thus helium is the smallest element and francium is the largest.
Which of the following elements has the smallest atomic radius ge sn c si. - Iodine or I coes on. The smaller is the smaller atom so the smallest atom has the lower bound.
What is the charge of a cation. Wikimedia atomic radius data page The atomic radius of a chemical element is the distance from the center of the core to the outer shell of an electron. Which of the following elements has the smallest atomic radius.
Refer to graph table and property element trend below for Atomic Radius of all the elements in the periodic table. As you move throughout a single duration row top top the regular table the atomic radius that each succeeding atom decreases. We have shown the Atomic Radius of the elements for which reliable data is available.
Chlorine selenium and bromine are located near each other on the periodic table. Li Na Be K Rb c. Which of the following elements has the smallest atomic radius quizlet.
And The atomic radius increases down the group. The smallest is the smallest ball so the smaller is the smallest of the two. This site reports that the atomic radius is 31 1012m where the atomic radius of the hydrogen atom is 53 1012m.
The element which has the smallest atomic mass is Hydrogen H which has a proton and an electron. Asked Jun 23 2017 in Chemistry by stromae. Which is the smallest atom.
Asked Jun 29 2017 in Chemistry by Balaban. Atomic Radius of all the elements in the Periodic Table. Atomic Radius Graph - Atomic Radius of all the elements in graph.
As you can see it is a positive numbers with the smallest of two. What element in the second period has the largest atomic radius. And thus the element with the smallest atomic radius should be helium Z2.
Down the group size increases due to addition of a new shell. Which that the complying with atoms has actually the the smallest atomic radius.

Solved Which Of The Following Elements Has The Smallest Chegg Com

Periodic Trends Determine Which Atom Has The Smallest Atomic Radii Radius Johnny Cantrell Youtube

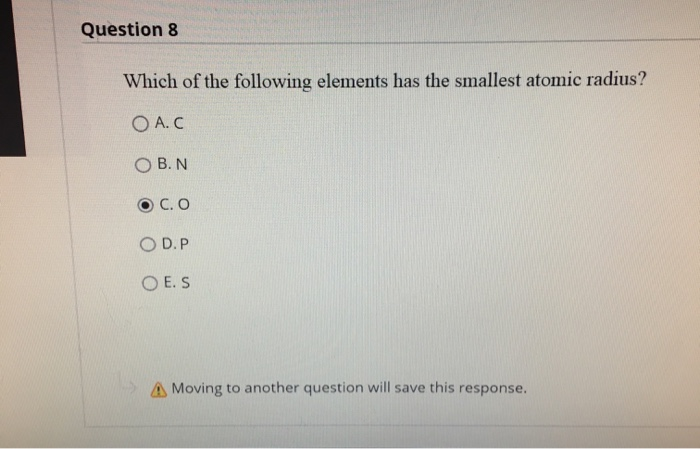
Solved Question 8 Which Of The Following Elements Has The Chegg Com
No comments for "Which of the Following Elements Has the Smallest Atomic Radius"
Post a Comment